November 2020 Case Study
by Azam Qureshi, MD
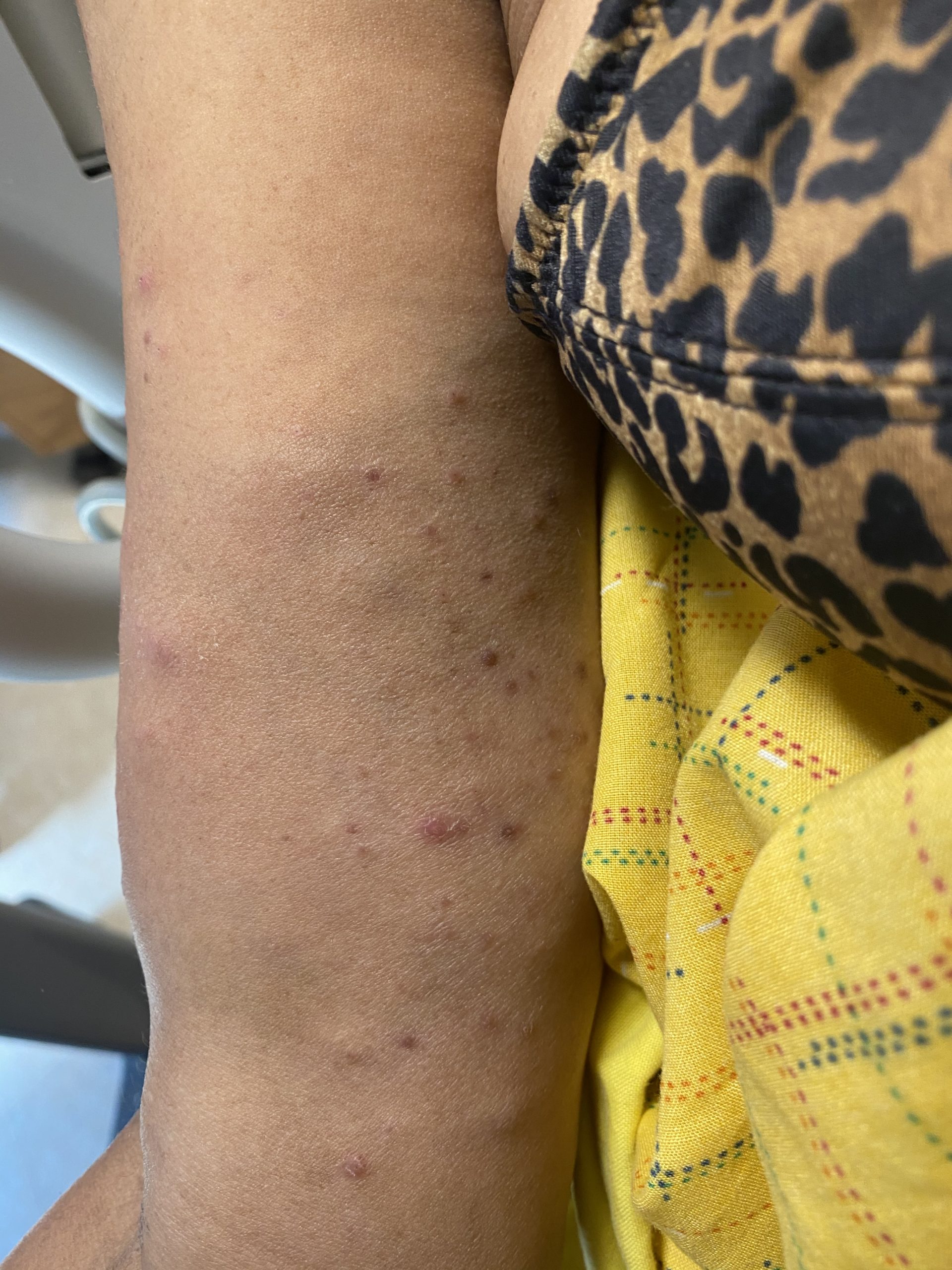
A 62-year-old female presents with a three week history of rash affecting the upper and lower extremities. On physical examination, subcutaneous nodules and multiple pink to purple scattered papules are noted on the distal upper extremities and the lower extremities extending from the outer thighs to the mid-anterior thighs bilaterally (Figures 1 & 2). The patient reports that some of the subcutaneous nodules are tender. She has not used any previous treatments. She has no family history of chronic diseases, including skin or autoimmune disease. She reports recent shortness of breath and mild cough, but otherwise, has been feeling well with no fevers, chills, night sweats, unexplained weight loss, abdominal pain, nausea, vomiting, diarrhea, headaches, or joint pains.
Blood work was notable for elevated erythrocyte sedimentation and chest x-ray was notable for only bilateral hilar lymphadenopathy. Two separate four millimeter punch biopsies were performed, and showed non-caseating epithelial granulomas throughout the superficial and deep dermis which lacked significant inflammatory rim of lymphocytes or plasma cells. Star-shaped eosinophilic inclusions of collagen were also seen. Brown-Hopps, Fite, and Gomori methenamine silver special stains were conducted on both biopsies and were negative for microorganisms.
Further investigation into the patient’s medical history is most likely to provide evidence for which of the following?
A.) Recent treatment of Hepatitis C with sofosbuvir & ledipasvir
B.) Recent spelunking in Kentucky
C.) Recent initiation of anti-retroviral therapy for treatment for HIV
D.) Recent travel from India
E.) History of major bowel surgery
Correct answer: C) Recent initiation of anti-retroviral therapy for treatment for HIV
This patient is presenting with sarcoidosis, as evidenced by the cutaneous morphologies of subcutaneous nodules and pink to purple papules affecting the upper and lower extremities, elevated erythrocyte sedimentation rate, pulmonary symptoms with bilateral hilar lymphadenopathy noted on chest x-ray, and skin biopsies showing evidence of “naked” epithelioid granulomas and asteroid bodies with negative Brown-Hopps, Fite, and Gomori methenamine silver special stains.
Anti-retrovirals in HIV patients have been shown to lead to possible drug-induced sarcoidosis.1,2 Furthermore, development of cutaneous sarcoidosis has been described as a feature of immune reconstitution syndrome in HIV patients initiating antiretroviral therapy.2 Although ribavirin and interferon-α treatment for hepatitis C have been associated with sarcoidosis onset, no such association has been made with the hepatitis C treatments of sofosbuvir or ledipasvir.3,4
Histoplasmosis (B) and cutaneous tuberculosis (D) may also present with pulmonary symptoms in conjunction with cutaneous manifestations and skin biopsy findings showing granulomatous inflammation. Sarcoidosis is the more likely diagnosis in this patient, as histoplasmosis presents more commonly with cutaneous molluscoid nodules, cellulitis, ulcers, panniculitis, and oral lesions. Skin biopsy would show intracellular 2-4 micron yeast with a surrounding halo within histiocytes. Cutaneous tuberculosis can present in a diverse manner, with histopathology showing evidence of suppurative inflammation, later becoming granulomatous with central caseous necrosis.
Cutaneous Crohn’s disease (E) can present with manifestations such as erythema nodosum, pyoderma gangrenosum, pyostomatitis vegetans, epidermolysis bullosa acquisita, and acrodermatitis enteropathica. There may be genital, perianal, or oral involvement. Cutaneous Crohn’s occurring away from the perianal, genital, or oral areas is rare, and more often affects the lower extremities and soles of the feet, with less common involvement of the upper extremities. Histopathology often shows non-caseating tuberculoid granulomas with an inflammatory rim of lymphocytes in the superficial and deep dermis, along with frequent Langerhans giant cells.
References
- Pascual JC, Belinchon I, Silvestre JF, Vergara G, Blanes M, Bañuls J, Betlloch I, Boix V. Sarcoidosis after highly active antiretroviral therapy in a patient with AIDS. Clinical and Experimental Dermatology: Clinical dermatology. 2004 Mar;29(2):156-8.
- Rachadi H, Ramli I, Meknassi I, Hassam B, Benzekri L. Recurrence of cutaneous sarcoidosis during immune reconstitution syndrome in an HIV-infected patient. InAnnales de dermatologie et de venereologie 2015 Dec (Vol. 142, No. 12, pp. 757-760).
- Hurst EA, Mauro T. Sarcoidosis associated with pegylated interferon alfa and ribavirin treatment for chronic hepatitis C: a case report and review of the literature. Archives of Dermatology. 2005 Jul 1;141(7):865-8.
- Cogrel O, Doutre MS, Marliere V, Beylot‐Barry M, Couzigou P, Beylot C. Cutaneous sarcoidosis during interferon alfa and ribavirin treatment of hepatitis C virus infection: two cases. British Journal of Dermatology. 2002 Feb;146(2):320-4.
- Bolognia JL, Schaffer JV, Duncan KO, Ko CJ. Dermatology essentials E-book. Elsevier Health Sciences; 2014 Feb 26.