February 2022 Case Study
by Kamaria Nelson, MD
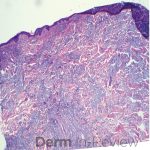
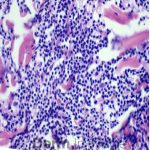
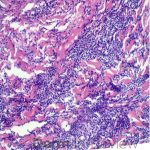
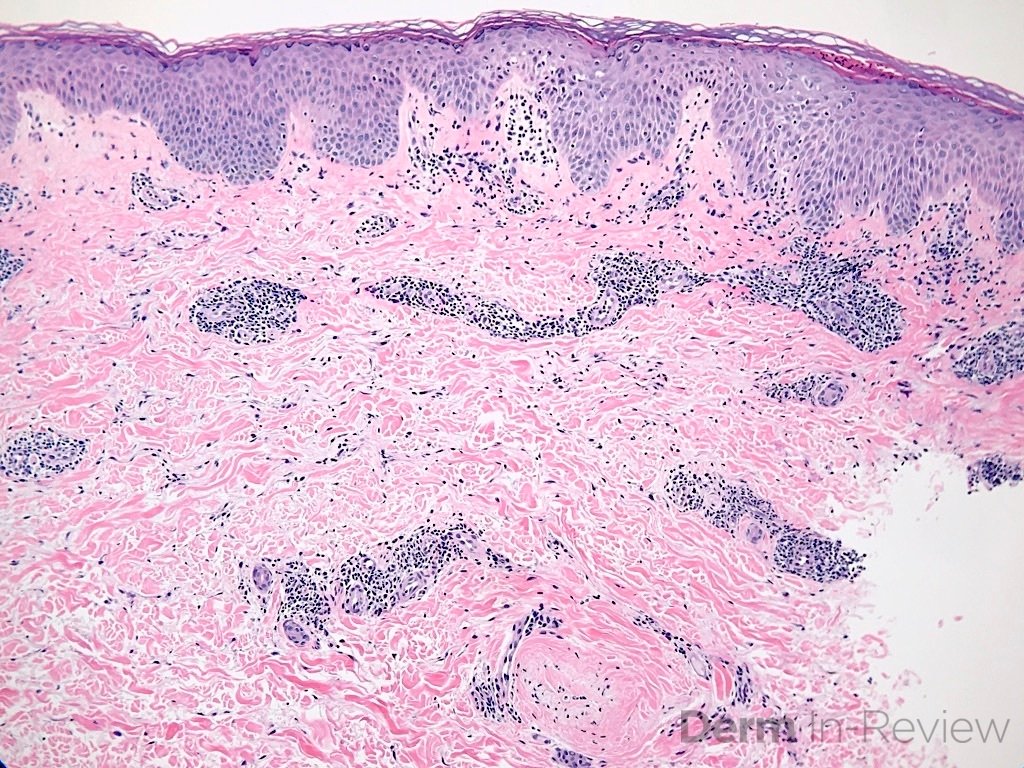
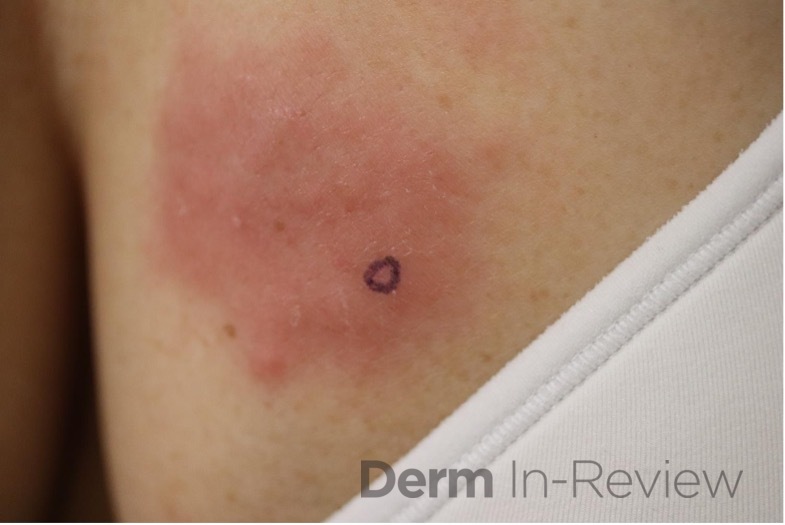
A 64-year-old female with no pertinent past medical history presents for a rash on the left breast. The rash has been present for about a month and started as a small red spot. The spot grew over the month and became firmer and more pruritic. She denies pain or bleeding or similar spots elsewhere on the body. She was treated with a mid-potency topical steroid with no improvement. On exam, on the left breast there is a well-defined firm pink plaque with scale (Image 1). A punch biopsy was performed (Images 2, 3, 4). Based on Immunohistochemical staining, a diagnosis of primary cutaneous marginal zone lymphoma was made.
Which of the following immunohistochemical staining results are consistent with this patient’s diagnosis?
A.) Bcl-2 -, Bcl-6 +, MUM-1 –
B.) Bcl-2 +, Bcl-6 -, MUM-1 –
C.) Bcl-2 +, Bcl-6 -, MUM-1 +
D.) Bcl-2 +, Bcl-6+, MUM-1+
Cutaneous B-Cell lymphomas are a rare group of non-Hodgkin lymphomas that primarily affect the skin. They are composed of cells with morphological characteristics of B cells normally found in the marginal zone or germinal centers of lymph nodes. Most are low-grade malignancies with good prognosis; however, aggressive variants do exist. There are 4 subtypes according to the WHO-EORTC Classification system: primary cutaneous follicular center lymphoma, primary cutaneous marginal zone B-cell lymphoma, primary cutaneous diffuse large B-cell lymphoma leg type, and intravascular diffuse large B-cell lymphoma.
Primary cutaneous follicular center lymphoma presents with a single or multiple papules, plaques or nodules with surrounding erythema in 1 anatomic region. Extracutaneous involvement is uncommon, and prognosis is excellent. Immunohistochemical staining will reveal Bcl-2 negative, Bcl-6 positive, and MUM-1 negative (answer A).
Primary cutaneous marginal zone B-cell lymphoma presents with solitary or multiple nodules or tumor on the upper body, trunk or extremities. Prognosis is excellent and 5-year survival is close to 100%. Histologically you will see a lymphocytic infiltrate in the dermis with marginal zone cells present. Immunohistochemical staining will reveal Bcl-2 positive, Bcl-6 negative and MUM-1 negative (correct answer B).
Primary cutaneous diffuse large B-cell lymphoma presents as solitary or localized red or purple papules, nodules, or plaques. When lesions present on the head and neck that indicates an excellent prognosis. However, when lesions present on the lower extremity, prognosis is poor with a 5-year survival of approximately 50%. MUM-1 is an immunohistochemical stain marker for the leg type (answers C and D). When MUM-1 stains positive that usually indicates a poorer prognosis.
For solitary or regional disease, radiation therapy or excision alone is recommended as initial treatment. Observation, topical therapies like topical steroids or intralesional steroids can be considered for some select patients, however there is limited data. Rarely, those that progress to extracutaneous disease will need treatment with chemotherapy or rituximab.
References
- Bolognia, J., Schaffer, J., & Cerroni, L. (2018). Dermatology (Fourth edition.). Philadelphia, Pa: Elsevier.
- James, Elston, D. M., Treat, J., Rosenbach, M. A., Neuhaus, I., & Andrews, G. C. (2020). Andrews’ Diseases of the Skin : Clinical Dermatology (Thirteenth edition.). Elsevier.
- Waldman RA, Finch J, Grant-Kels JM, Stevenson C, Whitaker-Worth D. Skin diseases of the breast and nipple: Benign and malignant tumors. J Am Acad Dermatol. 2019 Jun;80(6):1467-1481. doi: 10.1016/j.jaad.2018.08.066. Epub 2018 Nov 16. PMID: 30452954.
- Salemis NS, Koliarakis N, Spiliopoulos K, Kimpouri K, Marinos L. Primary cutaneous follicle center lymphoma of the breast: Management of an exceedingly rare malignancy. Intractable Rare Dis Res. 2020;9(4):263-265. doi:10.5582/irdr.2020.03095
- Malachowski SJ, Sun J, Chen PL, Seminario-Vidal L. Diagnosis and Management of Cutaneous B-Cell Lymphomas. Dermatol Clin. 2019 Oct;37(4):443-454. doi: 10.1016/j.det.2019.05.004. Epub 2019 Jul 30. PMID: 31466585.
- Lang CCV, Ramelyte E, Dummer R. Innovative Therapeutic Approaches in Primary Cutaneous B Cell Lymphoma. Front Oncol. 2020 Aug 7;10:1163. doi: 10.3389/fonc.2020.01163. PMID: 32850331; PMCID: PMC7426470.
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous B-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am J Hematol. 2020 Aug 20. doi: