February 2021 Case Study
by Jessica Kalen, MD
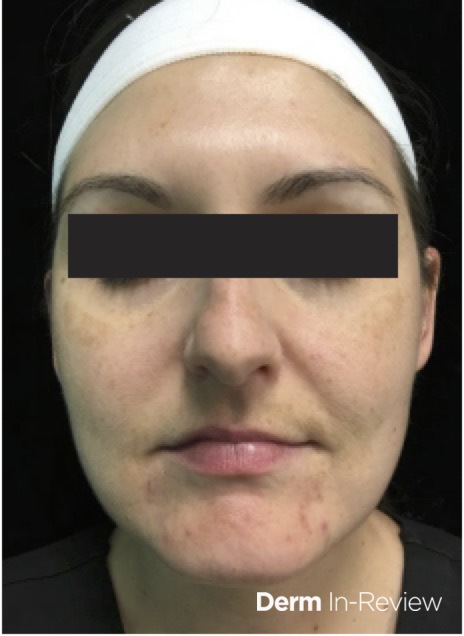
A 27-year-old-female with no past medical history presents for follow-up of acne. She was last seen three months ago where she was started on tretinoin cream nightly and benzoyl peroxide wash every morning. Despite use of topical medications, she reports continued flares of acne particularly around menses. On exam, she has erythematous to pink papules along the chin and jawline (Figure 1). Ultimately, she is started on spironolactone.
What is the appropriate lab monitoring for this patient?
A.) Comprehensive metabolic panel
B.) Liver function test
C.) Serum sodium
D.) Serum potassium only
E.) No lab monitoring required
Correct answer: E.) No lab monitoring required
Dermatologists frequently prescribe spironolactone to treat hormonal acne, as well as other off-label conditions. As an aldosterone antagonist, spironolactone primarily functions as a potassium sparing diuretic. However, spironolactone also has weak anti-androgenic properties and competitively inhibits androgen receptors, ultimately, halting biosynthesis of androgens.2
Spironolactone is FDA approved for management of hypertension, hypokalemia, primary hyperaldosteronism, and fluid retention resulting from cirrhosis and congestive heart failure. For dermatologic conditions, spironolactone is used as off-label therapy for hormonal acne, androgenic alopecia, hirsutism, and hidradenitis suppurativa.2 Side effects of this medication include gynecomastia, menstrual irregularities, hypotension, agranulocytosis, and hyperkalemia.
Use of spironolactone should be avoided in pregnancy due to theoretical risk of feminization of male fetuses.2 Additionally, spironolactone should be avoided in those with renal insufficiency, anuria, or Addison’s disease.2 Previously, it was thought that spironolactone should be avoided in patients with a personal history of breast, ovarian, or uterine case. However, a retrospective analysis revealed that use of spironolactone was not associated with increased risk of breast cancer recurrence in the two years following cancer treatment.3
Previously, due to perceived risk of hyperkalemia, many prescribers would monitor serum potassium levels of patients on spironolactone therapy. However, it has been shown that hyperkalemia occurs much less frequently than previously assumed.4 Thus, it has been suggested that healthy women under the age of 45 do not need routine serum potassium monitoring.5 Clinicians should consider routine serum potassium monitoring for women over the age of 45, those with cardiac or liver disease, or those taking other medications that can increase risk of hyperkalemia.5 In this clinical case, the patient is under the age of 45 with no other chronic medical conditions. Thus, the appropriate answer “no lab monitoring required.”
References
- Gold MH, et al. A cohort study using a facial cleansing brush with acne cleansing brush head and a gel cleanser in subjects with mild-to-moderate acne and acne-prone skin. Jour Drug Dermatol. November 2019;18(11):1140-1145.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy, 4th ed. Philadelphia. Elsevier.
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. May 2020;83(4):P1021-1027.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Thiede RM, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADA (Research of Adverse Drug Events and Reports) program. Int J Womens Dermatol. July 2019;5(3):155-157.