January 2023 Case Study
by Adam Rosenfeld, MD
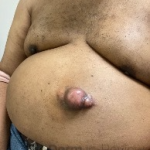
61-year-old male with history of HTN, DM, HLD who presented for a new growth on his mid abdomen. He noted a history of a trauma to the area with a metal blade 20 years prior while in active duty, with the area initially healing with a raised scar. He endorsed significant increase in size of the area over the last year, but it had been slowly growing for several years. The patient had been told this was a keloid. He denies any significant pain or associated symptoms. On examination, there was a large approximate 4x5cm protuberant skin colored to pink mass on the left mid abdomen (figure 1). A punch biopsy was obtained of this tumor demonstrating a dense dermal infiltrate of spindle cells (figure 2).
A diagnosis of a malignant dermal proliferation was made, often mimicking the diagnosis the patient had been told before. What stain was confirmatory in making this diagnosis?
A.) CD207+
B.) Factor XIIIA+
C.) S100+
D.) CD34+
E.) SMA+
- Figure 1
- Figure 2
Correct answer: D.) CD34+
This multi-loculated tumor is suspicious for dermatofibrosarcoma protuberans, or DFSP which classically stains diffusely positive for CD34. CD34 is a marker for vascular endothelium and hematopoietic progenitor cells4. It is positive in DFSP and negative in dermatofibromas. Dermatofibrosarcoma protuberans is a fibroblast derived intermediate-grade soft tissue sarcoma that can be mistaken for a keloid3. It is often slow growing and asymptomatic. It can present as a bulky multi-loculated tumor as in our patient but can also have much more innocent appearances. Its pathophysiology is unclear, but some have proposed prior injury to the skin as a triggering event1. Evidence does support these tumors arising from fibroblastic, histiocytic or neuroectoderm tissue although a fibroblastic origin seems most likely. The growth of the tumor is driven in most cases by a translocation of chromosome 17 and 22 forming the t(17,22) COLA1A-PDGFG fusion protein1. On histopathology, a dense dermal infiltrate of spindle cells is seen infiltrating into the deeper fat causing the “honey combing” appearance of the adipose tissue4. These cells stain diffusely positive for CD34+, while staining weakly or not at all for Factor XIIIA+ (answer choice B), the stain pathognomonic for dermatofibromas4. Importantly, it is crucial to rule out other spindle cell neoplasms including a spindle cell melanoma which would stain positive for S100 (answer choice C)4. While DFSPs are malignant, they typically do not demonstrate significant atypia histologically. Fibrosarcomatous change represents a subset of DFSPs that demonstrate much more atypical features, indicating tumor progression3. The differential diagnosis of DFSP includes but is not limited to a keloid, dermatofibroma, spindle cell melanoma, squamous cell carcinoma, Kaposi sarcoma.
The standard of care for treatment is surgical excision with every effort to achieve clear surgical margins both peripherally and deep2. Although wide local excision can be performed, Mohs Micrographic Surgery (MMS) is now recommended for these tumors. Given that it demonstrates unpredictable microscopic, tentacle like extensions, the directionality afforded by MMS makes it uniquely fit for DFSPs3. No set margins exist but wide local excision typically includes 2-4cm. MMS initial margins vary but the literature suggest 0.5-1.3cm3. Unresectable or metastatic tumors can be treated with radiation or Imatinib, a tyrosine kinase inhibitor5,7. NCCN guidelines do not recommended extensive systemic work up of these patients unless there are red flags for metastasis on exam or through history. Imaging with CT or MRI can be performed to assess for tumor depth but is based on a case-by-case basis8. These tumors have a low risk of metastasis (1-4%) but a high chance of local recurrence. Surveillance includes observation of primary site every 6 months for the first 5 years with yearly exams following3. The overall prognosis of DFSPS is good, with a 10-year survival rate of 99.1%3.
Incorrect stains in answer choices4
CD 207 (choice A) – surrogate marker for presence of Birbeck granules in Langerhans cells
Factor XIIIA (choice B) – stains dermal dendritic cells, positive in dermatofibroma and negative in DFSP
S100 (choice C) – stains melanocytes, Langerhans cells, sweat glands, nerves, Schwann cells, myoepithelial cells, fat, muscle and chondrocytes
SMA (choice E) – positive in smooth muscle, including vascular smooth muscle; negative in skeletal muscle; expressed in myofibroblasts and myoepithelial cells
References
- Bolognia, J., Schaffer, J., & Cerroni, L. (2018). Dermatology (Fourth edition.). Philadelphia,Pa: Elsevier.
- Dermatofibrosarcoma protuberans – statpearls – NCBI bookshelf. Dermatofibrosarcoma Protuberans. (n.d.). Retrieved August 11, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK513305/
- Hao X;Billings SD;Wu F;Stultz TW;Procop GW;Mirkin G;Vidimos AT; (n.d.). Dermatofibrosarcoma protuberans: Update on the diagnosis and treatment. Journal of clinical medicine. Retrieved August 11, 2022, from https://pubmed.ncbi.nlm.nih.gov/32516921/
- Elston, D. M. (2019). Dermatopathology. Elsevier.
- Cristian Navarrete-Dechent, M. D. (2019, March 1). Imatinib treatment for locally advanced or metastatic dermatofibrosarcoma protuberans. JAMA Dermatology. Retrieved August 11, 2022, from https://jamanetwork.com/journals/jamadermatology/article-abstract/2719525
- Raman K Madan, M. D. (2021, July 15). Dermatofibrosarcoma protuberans. Background, Pathophysiology, Etiology. Retrieved August 11, 2022, from https://emedicine.medscape.com/article/1100203-overview
- Ugurel, S., Mentzel, T., Utikal, J., Helmbold, P., Mohr, P., Pföhler, C., Schiller, M., Hauschild, A., Hein, R., Kämpgen, E., Kellner, I., Leverkus, M., Becker, J. C., Ströbel, P., & Schadendorf, D. (2014, January 16). Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: A multicenter phase II decog trial with long-term follow-up. American Association for Cancer Research. Retrieved August 15, 2022, from https://aacrjournals.org/clincancerres/article/20/2/499/78307/Neoadjuvant-Imatinib-in-Advanced-Primary-or
- Bichakjian, C. K., Olencki, T., Alam, M., Andersen, J. S., Berg, D., Bowen, G. M., Cheney, R. T., Daniels, G. A., Glass, L. F., Grekin, R. C., Grossman, K., Ho, A. L., Lewis, K. D., Lydiatt, D. D., Morrison, W. H., Nehal, K. S., Nelson, K. C., Nghiem, P., Perlis, C. S., … Ho, M. (2014, June 1).Dermatofibrosarcoma protuberans, version 1.2014. JNCCN. Retrieved January 9, 2023, from https://jnccn.org/view/journals/jnccn/12/6/article-p863.xml