February 2024 Case Study
Authors: Nagasai Adusumilli, MD, MBA1
1. Department of Dermatology, The George Washington University School of Medicine and Health Sciences
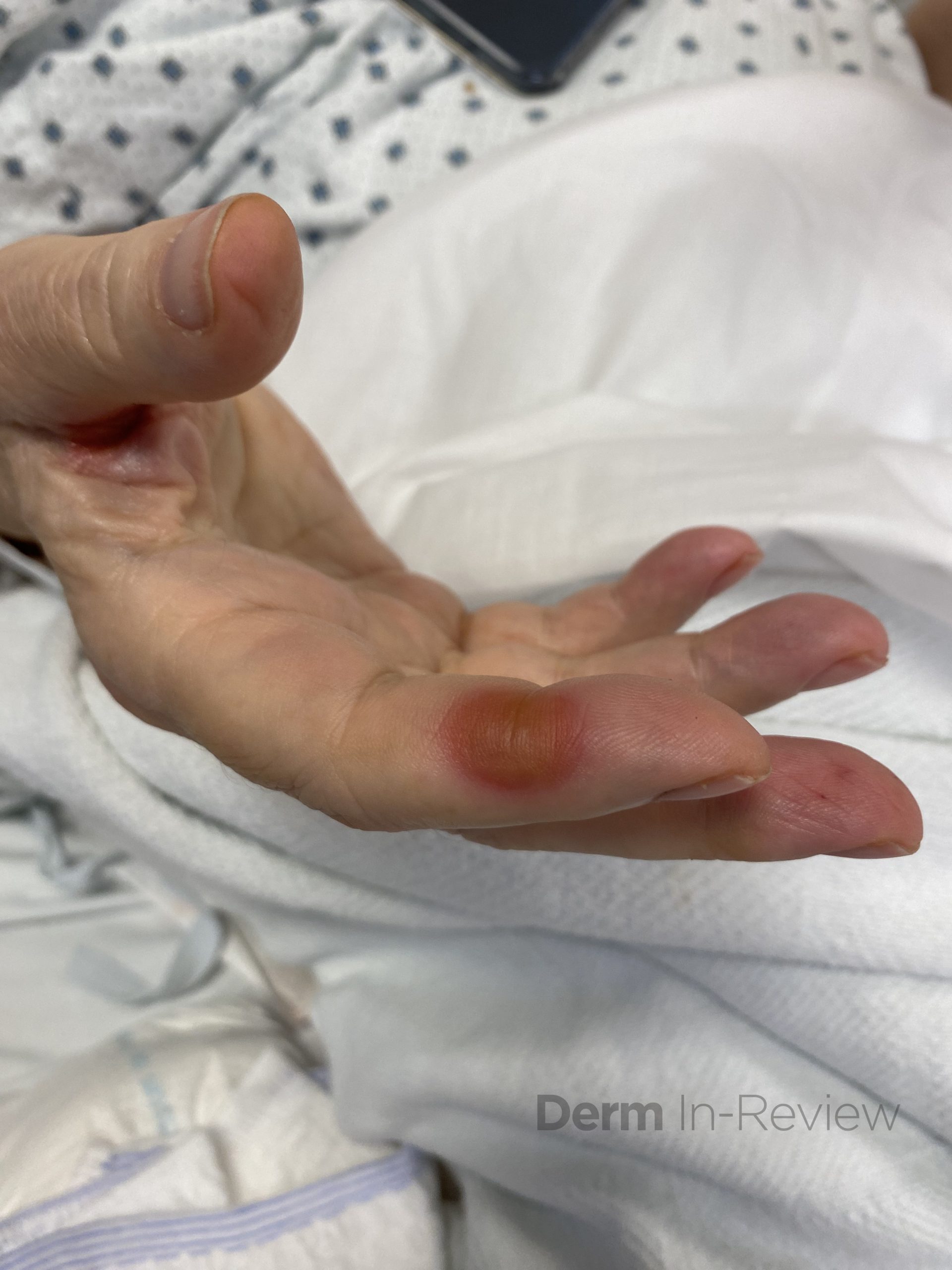
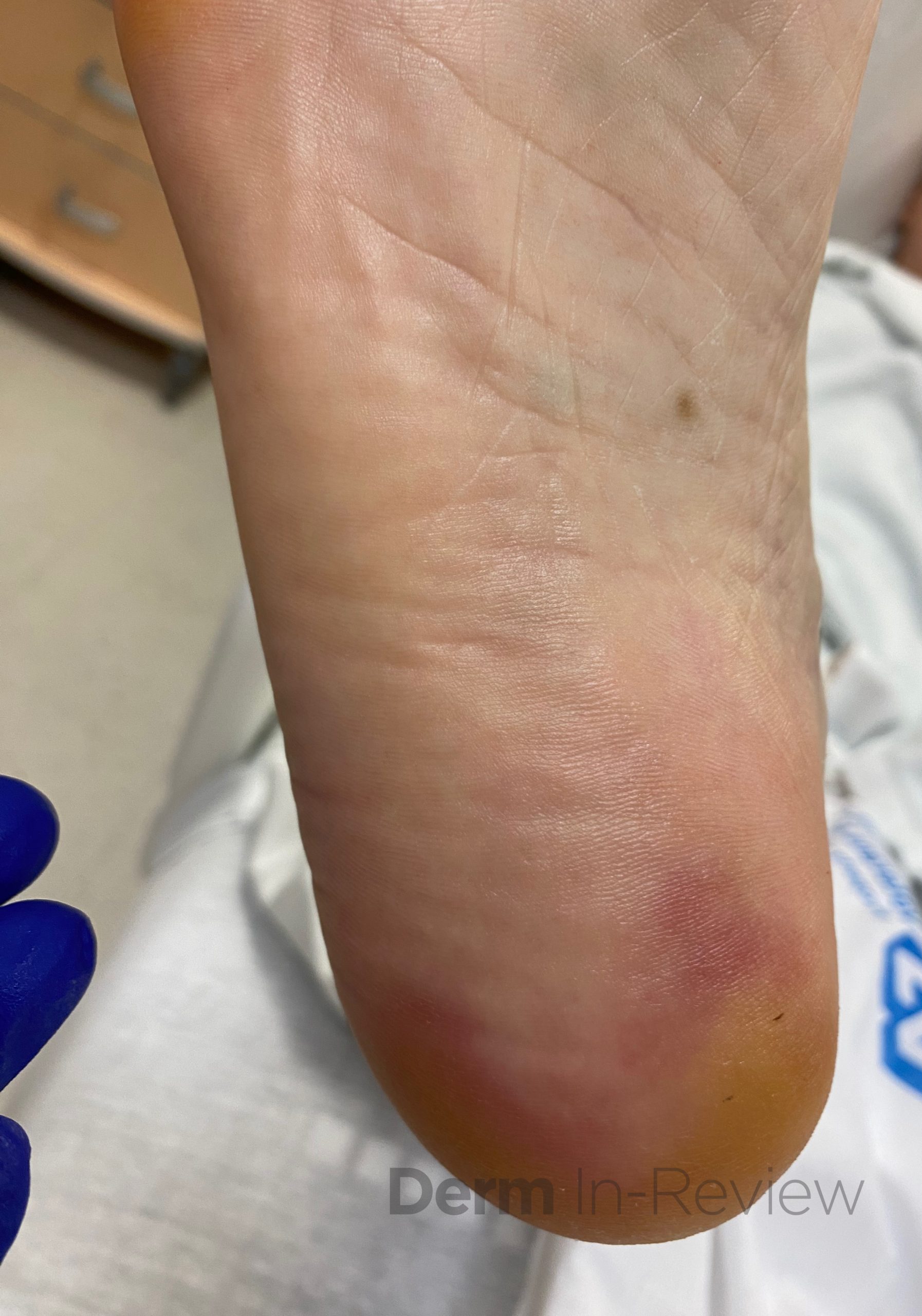
A 75-year-old male with a medical history of metastatic renal cell carcinoma, undergoing treatment with multiple systemic therapies, presented with 3 weeks of pink lesions on his hands, feet, and scrotum, with exquisite pain limiting ability to hold a pen and ambulate. Findings on the hands and feet are shown in Figure 1, and scrotal findings are shown in Figure 2. Notably, mucosa of the eyes, nose, mouth, urethral meatus, and anus were not involved. The patient experienced symptomatic improvement with 2 days of high potency topical corticosteroids and topical keratolytics.
Based on the clinical presentation, which of the following categories of medications has been most extensively implicated for this drug reaction?
A.) PD-1 inhibitors
B.) Beta-lactam antibiotics
C.) Aromatic anticonvulsants
D.) Sulfonamides
E.) Multikinase inhibitors

Correct Answer: E.) Multikinase inhibitors
Explanation of Correct Answer:
This patient’s clinical history and findings of multiple tender, pink-red thin macules and patches with a dusky center in the interdigital spaces of the hands, on the palmar surfaces of the fingers, and on the heels of the feet, along with scrotal desquamation, were consistent with hand-foot skin reaction (HFSR) and scrotal erythema, secondary to axitinib, a tyrosine kinase inhibitor of the vascular endothelial growth factor (VEGF) pathway. HFSR is classically characterized by discrete, exquisitely painful lesions with a halo of erythema, and hyperkeratotic lesions localized to sites of high contact, friction, and pressure, such as tips of fingers and toes, skin overlying metacarpophalangeal or interphalangeal joints, and heels.1,2 HFSR tends to develop within 2-4 weeks of starting multikinase inhibitors, such as sorafenib, sunitinib, cabozantinib, and axitinib (choice E), with pain that can impair range of motion, weight bearing, and overall function.1,2 Although scrotal involvement secondary to multikinase inhibitors is not as common, scrotal erythema, desquamation, and even ulceration have been reported.3
As newer immunotherapies and small molecule inhibitors are increasingly approved for more indications, identifying common and distinct drug reactions is integral to dermatology. Although skin reactions to any medication can be varied and atypical for individual patients, the morphology can be the key to diving deeper into the medical history to pinpoint the culprit, particularly as comorbidities and polypharmacy increase. Adverse skin eruptions from programmed cell death protein 1 (PD-1) inhibitors (choice A), such as pembrolizumab and nivolumab, more frequently present as morbilliform dermatoses, pruritus, lichenoid eruptions, psoriasiform dermatitis, and bullous pemphigoid.4 Although beta-lactam antibiotics encompass many medications, such as penicillins, cephalosporins, carbapenems, and monobactams, with a wide range of clinical presentations for drug reactions, the class overall is one of the most common causes of acute generalized exanthematous pustulosis5 (choice B). Aromatic anticonvulsants, such as phenytoin, carbamazepine, and phenobarbital (choice C) are commonly implicated in drug induced hypersensitivity syndrome (DIHS), previously drug reaction with eosinophilia and systemic symptoms (DRESS), and in Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).5 Similarly, drug reactions from sulfonamides (choice D) have multiple possible presentations, but exanthematous eruption, fixed drug eruption, and SJS/TEN are the most characteristic.5
HFSR is often used interchangeably with palmoplantar erythrodysthesia, or hand-foot syndrome, which was initially described as a subset of toxic erythema of chemotherapies, such as taxanes, cytarabine, capecitabine, anthracyclines, and systemic 5-fluorouracil. Although HFSR can clinically appear similar to hand-foot syndrome, they are separate drug reactions, with the localized hyperkeratotic lesions with a rim of erythema distinct from the symmetric erythema, edema, paresthesia, and dysesthesia seen with hand-foot syndrome from cytotoxic chemotherapies. A high density of eccrine glands is thought to drive the skin manifestations of hand-foot syndrome5 whereas inhibited vascular repair mechanisms in fibroblasts and endothelial cells via VEGF and platelet-derived growth receptor (PDGFR) are hypothesized to predispose areas of trauma to HFSR.6 In fact VEGF and PDGFR inhibitors, such as sorafenib, sunitinib, cabozantinib, and axitinib are among the multikinase inhibitors reported to cause HFSR.1,3,6
Contrary to the nomenclature, the tender erythematous patches and plaques are not limited to just the hands and feet. Flexural surfaces and bony prominences can also be affected. Identifying that each figure of this case illustrates areas of high friction or pressure is key to clinching the diagnosis, even without knowing the specific medications that can treat metastatic renal cell carcinoma. Grading the severity of cutaneous adverse events dictates the overall management strategy.7 Because these symptoms are limiting self-care activities of daily living, this patient has a grade 3 cutaneous adverse event,7 warranting collaboration with oncology colleagues to consider dose reduction, discontinuation, or temporary cessation of the causative anticancer agent.1 In the interim, high potency topical corticosteroids and topical keratolytics, such as urea cream, may provide symptomatic relief.1,8
References:
- Lacouture ME, Wu S, Robert C, at al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008 Sep;13(9):1001-11. PMID: 18779536.
- Kaul S, Kaffenberger BH, Choi JN, Kwatra SG. Cutaneous adverse reactions of anticancer agents. Dermatol Clin. 2019 Oct;37(4):555-568. PMID: 3146695.
- Zuo RC, Apolo AB, DiGiovanna JJ, et al. Cutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinib. JAMA Dermatol. 2015 Feb;151(2):170-7. PMID: 25427282.
- Adusumilli NC, Friedman AJ. Treating cutaneous immune-related adverse events from immune checkpoint blockade. J Drugs Dermatol. 2021 Oct 1;20(10):1133-1134. PMID: 34636526.
- Valeyrie-Allanore L, Obeid G, Revuz J. Chapter 21 – Drug Reactions. In: Dermatology. Fourth Edition. Elsevier; 348-375.
- Fischer A, Wu S, Ho AL, Lacouture ME. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Investigational new drugs. 2013;31(3):787-797. PMID: 23345001.
- NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 data files. 2017. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 20 Jan 2024.
- Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015 Mar 10;33(8):894-900. PMID: 25667293.

