March 2022 Case Study
by Daniel Nussbaum, MD
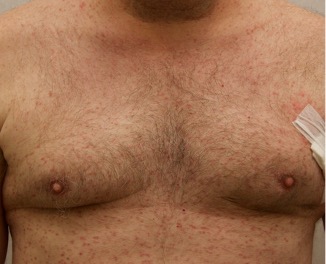
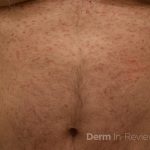
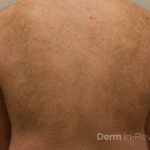
A 50-year-old man with no past medical history presents with the pictured rash on his chest, arms, abdomen, and back for five years. Although asymptomatic, the patient reported increasing redness under hot water without associated pruritus. He denied any systemic symptoms. Examination showed monomorphic erythematous macules and papules distributed diffusely over the chest, upper extremities, abdomen, and back. He denies any systemic symptoms and has no personal or family history of skin conditions other than the pictured lesions.
Which of the following laboratory tests would aid in confirming the diagnosis in this patient?
A.) Indirect immunofluorescence
B.) Anti-gliadin Ab
C.) Anti-nuclear Ab
D.) Anti-Jo-1 Ab
E.) Serum tryptase
Correct answer: E
The history and image provided are most consistent with a adult onset cutaneous mastocytosis. Histopathology of cutaneous mastocytosis commonly reveals mast cells concentrated in the dermal papillae, which can be confirmed with staining for mast cells (see below).1 The patient was recommended to avoid triggers for mast cell degranulation such as heat, sunlight, stress, medications, and alcohol. Additionally, he was referred to hematology for bone marrow biopsy, which was deferred due to lack of systemic symptoms.
Mastocytosis is a spectrum of diseases with various cutaneous and systemic manifestations. Cutaneous findings include flushing, pruritus, urticaria, and bullae. Systemic symptoms include bone pain, headaches, fatigue, nausea, vomitus, diarrhea, weight loss, cramping, chest pain, palpitations, and syncope.1 Patients with mastocytosis, especially the systemic variant, frequently have a mutation in KIT D816V, a tyrosine kinase receptor. Tissue diagnoses of mastocytosis can be confirmed with Giemsa, CD117, Leder, toluidine blue, and tryptase stains.
Diffuse cutaneous mastocytosis is a rare subset of mastocytosis, usually presenting in childhood low risk of systemic involvement. However, when presentation occurs in adulthood, systemic involvement should be evaluated with serum tryptase and bone marrow biopsy when indicated. Without a bone marrow biopsy, the risk of systemic involvement can be estimated from symptoms and a tryptase level.2 Based on clinical presentation and laboratory findings, physicians must consider working up systemic involvement to avoid morbidity and mortality.3,4
Indirect immunofluorescence (IIF) (answer A) is useful in the diagnosis of blistering dermatoses. Specifically, IIF of serum Ab to monkey esophagus can be useful in the diagnosis of pemphigus vulgaris.5 Anti-gliadin Ab (answer B) are commonly found in patients with dermatitis herpetiformis, which is a skin manifestation of gluten-sensitive enteropathy, commonly known as celiac disease.6 Anti-nuclear Ab (answer C) are non-specific for various autoimmune conditions such as systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, among others.7 Anti-Jo-1 Ab (answer D) are more specific autoimmune markers for patients with dermatomyositis and polymyositis.8 [/vc_column_text]
References
- Bolognia JK, Schaffer JV, Cerroni L. Dermatology. Philadelphia: Elsevier. 2018.
- Fuchs D, Kilbertus A, Kofler K, et al. Scoring the risk of having systemic mastocytosis in adult patienst with mastocytosis in the skin. J Allergy Clin Immunol Pract. April 2021;9(4):1705-1712.
- Mannelli F. Catching the clinical and biological diversity for an appropriate therapeutic approach in systemic mastocytosis. Ann Hematol. 2021;100:337-344.
- Pardanani, A. Systemic mastocytosis in adults: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2021;96:508-525.
- Kridin K, Bergman R. The Usefulness of Indirect Immunofluorescence in Pemphigus and the Natural History of Patients With Initial False-Positive Results: A Retrospective Cohort Study. Front Med (Lausanne). 2018;5:266.
- Benson BC, Mulder CJ, Laczek JT. Anti-gliadin antibodies identify celiac patients overlooked by tissue transglutaminase antibodies. Hawaii J Med Public Health. 2013;72(9 Suppl 4):14-17.
- Heffernan MP, Do JH, Mehta J. Antinuclear antibodies in dermatology. Semin Cutan Med Surg. 2001;20(1):2-13.
- Mileti LM, Strek ME, Niewold TB, Curran JJ, Sweiss NJ. Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience. J Clin Rheumatol. 2009;15(5):254-255.