April 2025 Case Study
Author: Nikita Menta, BA1, Nidhi Shah, MD1, Karl Saardi, MD1
- Department of Dermatology, The George Washington University School of Medicine and Health Sciences
Patient History
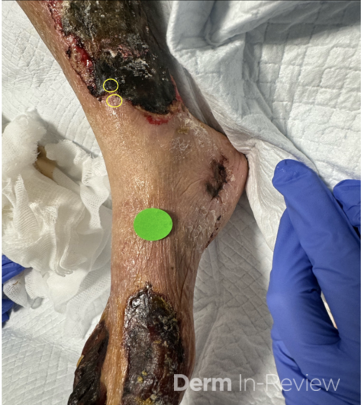
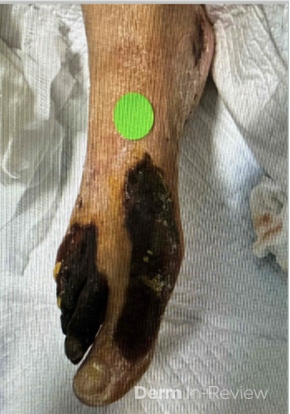
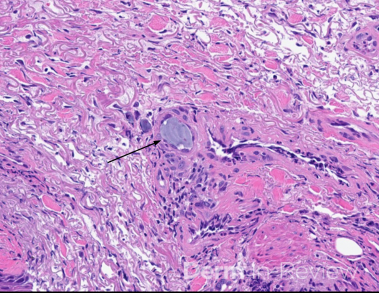
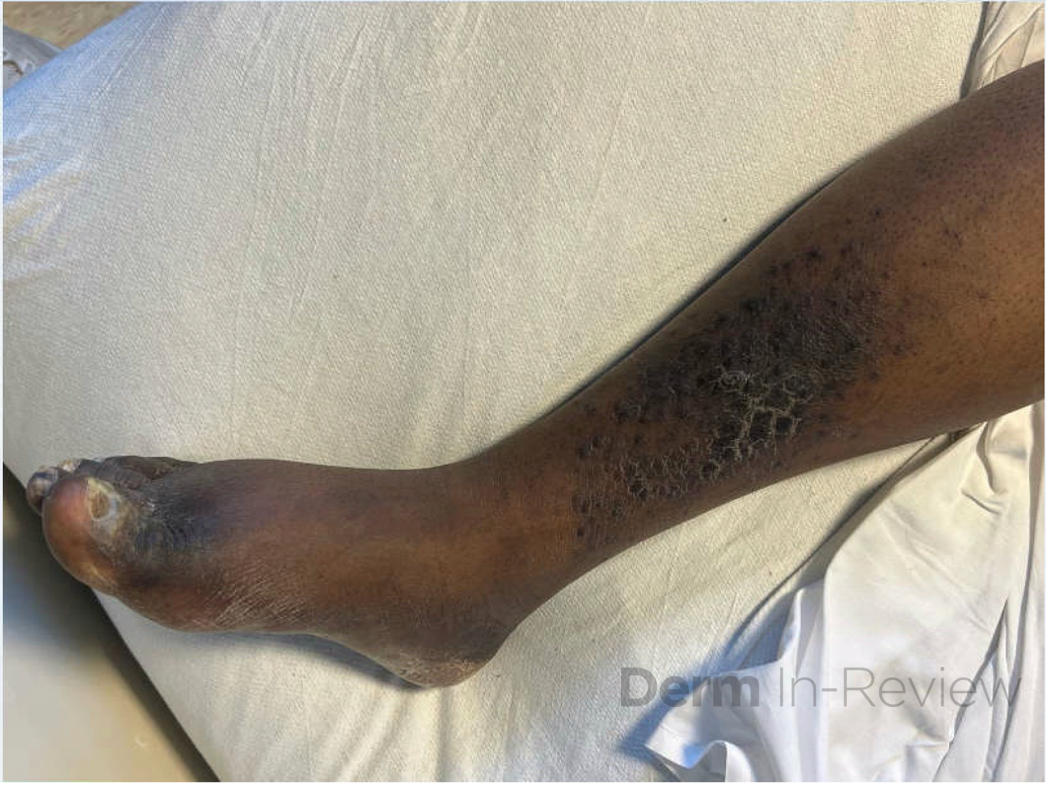
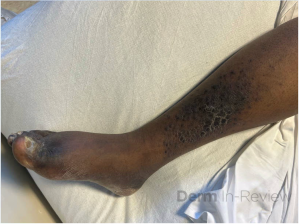
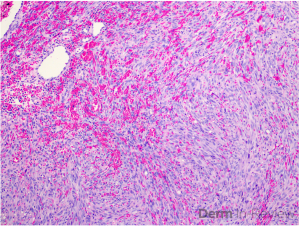
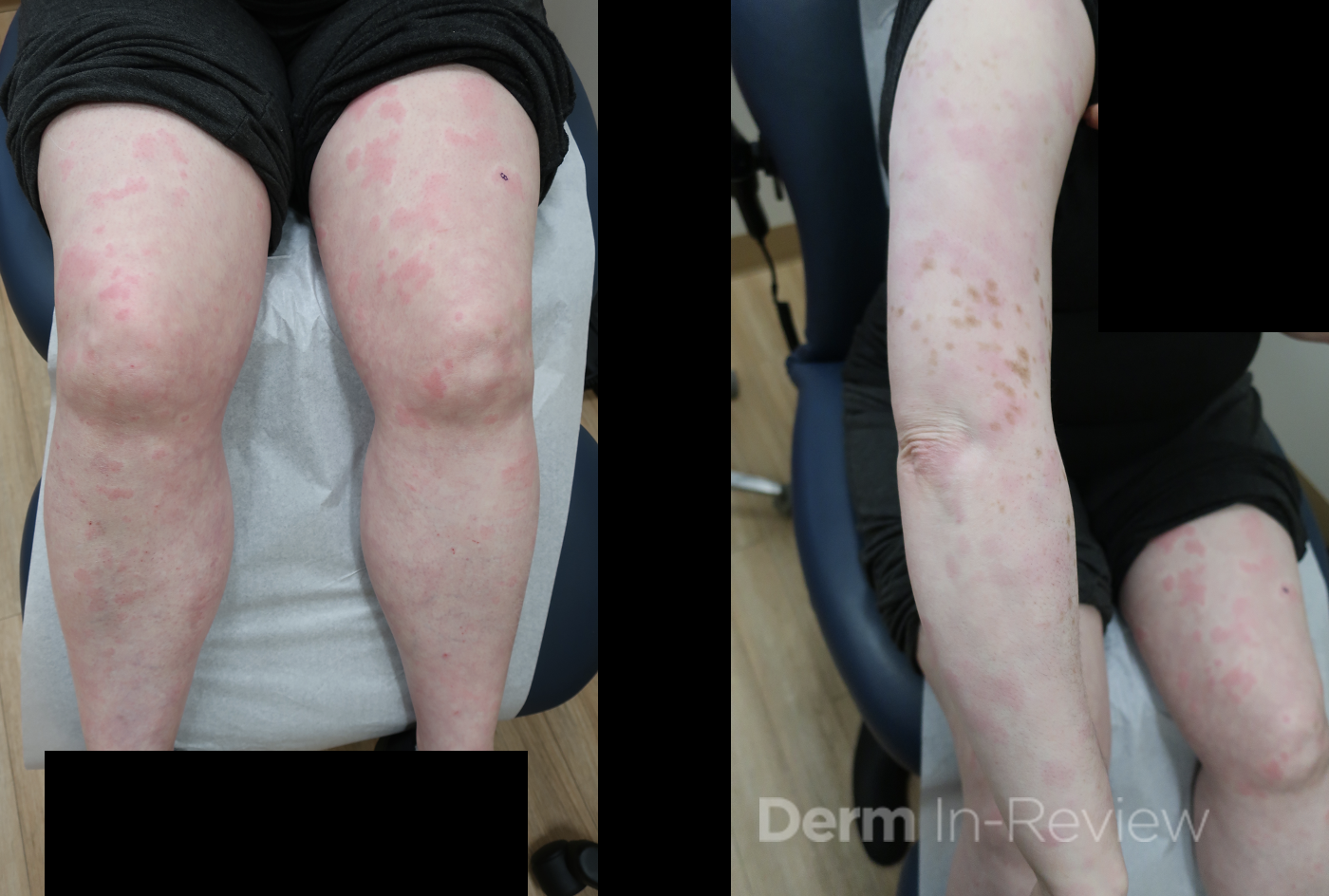
A 43-year-old female presented to the clinic with several painful, erythematous, edematous plaques in addition to hyperpigmented plaques on the arms and legs, which had been present for more than 24 hours. The rash initially started about four years prior to presentation and was unresponsive to a variety of oral antihistamines and a four-month trial of omalizumab. The patient also endorsed intermittent episodes of angioedema as well as occasional low back and knee pain. She previously underwent a punch biopsy on the lower left back; hematoxylin and eosin staining demonstrated marked dermal edema, margination of neutrophils with perivascular and interstitial infiltrate including eosinophils, perivascular nuclear debris with neutrophils, and significant extravasation of erythrocytes. Exam findings are shown in Figure 1.
Based on the clinical presentation and biopsy findings, which of the following laboratory tests would be most useful in determining the prognosis of this condition?
A) Antinuclear antibodies
B) Erythrocyte sedimentation rate
C) Complement Levels
D) C-reactive protein
E) Liver function tests
F) Rheumatoid factor
G) Antithyroid antibodies
Correct Answer: C
Explanation/Literature Review:
This patient’s treatment-resistant urticarial plaques combined with histopathologic findings demonstrating perivascular nuclear debris with neutrophils and extravasation of erythrocytes are consistent with a diagnosis of urticarial vasculitis (UV). UV classically presents as recurrent, long-lasting wheal-like lesions with a predilection for the trunk and proximal extremities, accompanied by histopathologic findings of leukocytoclastic vasculitis.1 UV can present with or without angioedema. Additionally, features that help distinguish UV from chronic spontaneous urticaria (CSU) include the persistence of lesions beyond 24 hours, predominant symptoms of burning and pain compared to pruritus, and residual post-inflammatory purpura or hyperpigmentation upon healing.2,3 UV is a rare condition with an estimated incidence of 0.5 per 100,000 in the United States.4 It most commonly occurs in individuals in their 5th to 7th decades of life and demonstrates a female gender predominance.5 Regarding pathophysiology, UV is a type III hypersensitivity reaction; however, in most cases, the triggering antigen is often unidentified.6 While the majority of UV cases are idiopathic, a small percentage of cases have been associated with medications, infectious diseases, malignancies, and autoimmune diseases, commonly systemic lupus erythematosus.2
There are two main subtypes of UV, which are classified based on complement levels, the most important indicator of UV prognosis (answer choice C). Patients with normal complement levels, or normocomplementemic UV (NUV), tend to have skin-limited vasculitis. Conversely, patients with low complement levels, or hypocomplementemic UV (HUV), are much more likely to experience systemic disease.1,2 The most common systemic manifestations of HUV include arthralgia, arthritis, inflammatory eye diseases, pulmonary symptoms (mimicking asthma or COPD), gastrointestinal symptoms, and renal pathologies (proteinuria/hematuria).1 Finally, there is also a more severe subtype of HUV, called HUV syndrome (HUVS), which is defined by multiorgan involvement.1
UV is a notoriously challenging condition to treat, further complicated by the lack of standardized guidelines and randomized controlled trials. In a recent review article, H1-antihistamines, omalizumab, and immunomodulators were among the treatments with the most robust evidence in UV management.4,7 However, while H1-antihistamines were widely employed, they were only effective in improving skin lesions in 9.9% of 141 patients.7 Omalizumab demonstrated much greater efficacy, with 76% of 108 patients experiencing improvement in cutaneous symptoms and many also experiencing improvement in systemic symptoms such as arthralgia and abdominal pain.7 Among immunomodulators, systemic corticosteroids, dapsone, and hydroxychloroquine had the most substantial and strongest evidence supporting their use in addressing cutaneous and systemic symptoms of UV; however, the potential adverse effects of these drugs must be carefully considered.4,7 The aforementioned review article proposed a treatment algorithm recommending that mild UV should be approached like CSU, starting with H1-antihistamines and escalating to omalizumab, whereas moderate-to-severe UV should be managed with corticosteroids and/or other immunomodulatory agents depending on the patient profile.7 While this algorithm offers some direction, there is a critical need for high-quality, head-to-head trials to determine the optimal approach to UV management.
Incorrect answer choices:
The other laboratory tests are not indicative of prognosis but are warranted in the workup of patients with UV, particularly HUV. Inflammatory markers, specifically erythrocyte sedimentation rate (B) and C-reactive protein (D), are typically elevated in UV.1,5 Additionally, since UV can be associated with autoimmune diseases, autoimmune laboratory tests, including but not limited to antinuclear antibodies (A), rheumatoid factor (F), and anti-thyroid antibodies (G) are often included in the workup to detect or rule out underlying autoimmune diseases.5 Finally, while liver dysfunction is not a common systemic manifestation of UV, liver function tests (E) may be included alongside standard laboratory tests, such as complete blood count and basic metabolic panel, in the workup of UV.5
References
- Marzano AV, Maronese CA, Genovese G, et al. Urticarial vasculitis: Clinical and laboratory findings with a particular emphasis on differential diagnosis. J Allergy Clin Immunol. 2022;149(4):1137-1149. doi:10.1016/j.jaci.2022.02.007
- Bolognia J, Schaffer JV, Cerroni L, Callen JP. Dermatology. Elsevier; 2025.
- James W. Andrews’ Diseases of the Skin. Elsevier; 2026.
- Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: A systematic review. J Allergy Clin Immunol. 2019;143(2):458-466. doi:10.1016/j.jaci.2018.09.007
- Wang RX, Newman SA. Urticarial Vasculitis. Immunol Allergy Clin North Am. 2024;44(3):483-502. doi:10.1016/j.iac.2024.03.006
- Black AK. Urticarial vasculitis. Clin Dermatol. 1999;17(5):565-569. doi:10.1016/s0738-081x(99)00062-0
- Rothermel ND, Vera Ayala C, Gonçalo M, et al. Managing Urticarial Vasculitis: A Clinical Decision-Making Algorithm Based on Expert Consensus. Am J Clin Dermatol. 2025;26(1):61-75. doi:10.1007/s40257-024-00902-y