November 2022 Case Study
by Erika McCormick, BS, Emily Murphy, MD, Adam Friedman, MD
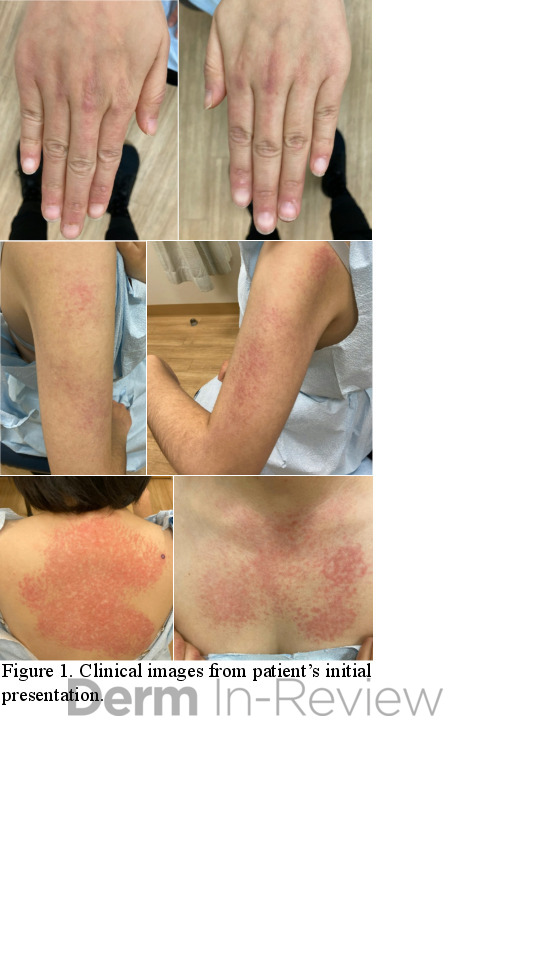
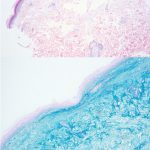
A 42-year-old female presented with a rash of two years duration affecting the hands, upper arms, upper back, and chest (Figure 1). A punch biopsy from the back was performed (Figure 2). Laboratory analysis was significant for transcriptional intermediary factor (TIF)-1γ antibody positivity.
Which of the following has been associated with TIF-1γ antibody positivity in this condition?
A.) Mild or absent muscle disease
B.) Increased risk of malignancy
C.) Decreased risk of malignancy
D.) Increased risk of interstitial lung disease
E.) Immune-mediated necrotizing myopathy
Correct answer: B.) Increased risk of malignancy
This patient presented with skin changes consistent with dermatomyositis (DM), including erythematous papules overlying the knuckles (Gottron papules), photodistributed poikiloderma involving the chest (‘V-neck’ sign) and upper back (‘shawl’ sign), and erythema surrounding the periocular region (heliotrope rash; not pictured).2 Punch biopsy from the back showed vacuolar interface dermatitis with necrotic keratinocytes and increased superficial dermal mucin by colloidal iron staining, consistent with DM. A myositis specific antibody panel was performed. Myositis-specific autoantibodies (MSAs) are found in approximately 60 to 70% of children and adults with idiopathic inflammatory myopathy (encompasses DM, inclusion body myositis, polymyositis, immune-mediated necrotizing myopathy, and anti-synthetase syndrome).3 MSAs tend to be mutually exclusive, therefore their detection on laboratory analysis can help identify distinct subsets of disease, predict clinical phenotype, and stratify risk for malignancy and interstitial lung disease.3
In adults greater than 40 years old with DM, anti-TIF1γ autoantibody positivity has a strong association with increased risk of malignancy.3 (Choice B) Anti-nuclear matrix protein 2 (NXP2) is the second most common MSA seen in DM patients with malignancy.3,4 Conversely, antibodies to Mi2 are associated with decreased risk of malignancy.3 (Choice C) Prevalence of malignancy in DM is estimated at 15% to 25%2 with greatest risk within the first 3 years of diagnosis.5 Studies suggest that over 50% of anti-TIF1γ antibody positive DM patients may develop malignancy,6,7and that risk increases to nearly 75% if anti-TIF1α antibodies are also present.6 Conventional recommendations upon diagnosis of DM with elevated malignancy risk include CT scan of the chest, abdomen, and pelvis in addition to obtaining thorough medical history, physical exam, laboratory tests, and age-appropriate cancer screenings.2,8–10 Positron emission tomography with fluorodeoxyglucose and CT (FDG-PET/CT) was found to be comparable to CT alone but may be associated with an increased biopsies without improved diagnostic yield.9,11
DM patients with anti-TIF1 autoantibodies typically have extensive skin involvement but have decreased risk of interstitial lung disease (ILD). 12 (Choice D) Antibodies associated with increased prevalence and severity of ILD include those to aminoacyl-tRNA synthetases (especially PL7, PL12, KS, Jo1, and OJ), PM/Scl, and melanoma differentiation associated protein 5 (MDA-5).3 In anti-MDA-5 antibody positive DM, ILD prevalence ranges from 50% to 100% and can be rapidly progressive with high mortality.13 MDA-5 antibodies are also associated with a characteristic phenotype of mucocutaneous ulcerations, arthritis, and mild or absent muscle disease.3 (Choice A) Immune-mediated necrotizing myopathy, characterized by severe muscle disease refractory to conventional immunosuppressive therapies,3 is associated with antibodies to signal recognition particle (SRP) and to 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR).3 (Choice E)
The treatment approach for DM varies based on severity and extent of myositis; however, it generally includes systemic corticosteroids and immunosuppressive drugs. This patient received a prednisone taper, topical clobetasol 0.05% ointment for rash on the body, and topical hydrocortisone 2.5% ointment for the face. After failing methotrexate, hydroxychloroquine, and mycophenolate mofetil, this patient was successfully transitioned to high dose IVIG. A CT scan of the chest, abdomen, and pelvis was negative for any signs of malignancy and the patient continued age-appropriate cancer screenings with her primary care physician.
References
- Wolstencroft PW, Rieger KE, Leatham HW, Fiorentino DF. Clinical factors associated with cutaneous histopathologic findings in dermatomyositis. J Cutan Pathol. 2019;46(6):401-410.
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Fourth edition. (Bolognia J, Schaffer JV, Cerroni L, eds.). Elsevier; 2018.
- McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol. 2018;14(5):290-302.
- Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 2013;65(11):2954-2962.
- Kardes S, Gupta L, Aggarwal R. Cancer and myositis: Who, when, and how to screen. Best Pract Res Clin Rheumatol. 2022;36(2).
- Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64(2):513-522.
- Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford). 2010;49(9):1726-1733.
- Oldroyd AGS, Allard AB, Callen JP, et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60(6):2615-2628.
- Maliha PG, Hudson M, Abikhzer G, Singerman J, Probst S. 18F-FDG PET/CT versus conventional investigations for cancer screening in autoimmune inflammatory myopathy in the era of novel myopathy classifications. Nucl Med Commun. 2019;40(4):377-382.
- Vaughan H, Rugo HS, Haemel A. Risk-Based Screening for Cancer in Patients With Dermatomyositis: Toward a More Individualized Approach. JAMA Dermatol. 2022;158(3):244-247.
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123(6):558-562.
- Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449-455.
- Wu W, Guo L, Fu Y, et al. Interstitial Lung Disease in Anti-MDA5 Positive Dermatomyositis. Clin Rev Allergy Immunol. 2021;60(2):293-304.